Please join us in congratulating Simpson Lab student researchers, Jessica…
Simpson Lab Publishes First Research Paper on Darier Disease
Originally published to the UW Division of Dermatology website on August 17, 2023.
A new study by the Simpson Lab shows MEK inhibitors, used to control the growth of cancer cells like melanoma, may be the key to reducing skin breakdown in patients with Darier disease, a rare genetic skin condition characterized by blistering lesions and recurrent skin infections. The findings are summarized in an article titled, “Targeting SERCA2 in organotypic epidermis reveals MEK inhibition as a therapeutic strategy for Darier disease,” published online on August 10, 2023, by the journal JCI Insight.
This is the first primary research paper published by the Simpson Lab, established by Cory Simpson, MD, PhD, after joining the University of Washington Division of Dermatology in 2021. The lab is affiliated with the UW Division of Dermatology and UW Medicine Institute for Stem Cell and Regenerative Medicine.
“This paper is a big milestone for my group and we are very excited to see the story published after a lot of hard work,” says Dr. Cory Simpson. “Beyond the exciting clinical implications of the science, it also represents successful teamwork over the past two years, both within my lab and also with wonderful collaborators from Penn and Michigan.”
The study was conducted by lead author Shivam Zaver, MD, PhD, 2023 University of Washington Medical Student Training Program graduate, and co-author Afua Tiwaa, Research Scientist, with guidance from senior author, Cory Simpson, MD, PhD, Assistant Professor and Principal Investigator for the Simpson Lab.
Additional collaborators include Mrinal Sarkar, PhD, and Johann Gudjonsson, MD, PhD, from the University of Michigan Department of Dermatology, and Shaun Egolf, PhD, Jonathan Zou, and Brian Capell, MD, PhD, from the University of Pennsylvania Perelman School of Medicine Department of Dermatology.
Congratulations to the Simpson Lab Research Team and collaborators!
Research Summary
To begin identifying potential therapies for Darier disease, researchers had to establish a human tissue model to better understand how the pathology develops. Darier disease is a rare genetic skin blistering disorder with no current FDA-approved therapies. The model was created using CRISPR/Cas9 (gene-editing technology) to engineer human keratinocytes (the cells that make up our outer skin barrier, the epidermis), and disrupting the disease-linked gene ATP2A2, which encodes a calcium pump (SERCA2) that resides in the endoplasmic reticulum.

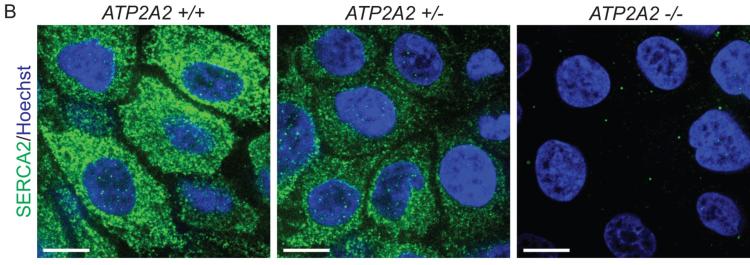
“Research into Darier disease has been slowed by the fact that targeting the involved gene in mice does not replicate the skin blistering seen in patients. So, researchers have not had what we call a pre-clinical model, which is essential for testing hypotheses about how the disease works and trying out potential treatments in the lab before involving patients. We used gene editing technology in an organoid model of the skin, allowing us to mimic the genetics of Darier disease in 3D human epidermal tissue that we can generate for experiments in the lab.”
Using RNA sequencing, proteomics, and confocal microscopy to analyze the cells, researchers found up-regulation of signaling through MEK, which operates in the MAP kinase pathway, a major regulator of cell growth, differentiation, and adhesion.
The Simpson Lab’s findings show that in skin biopsies from patients with Darier disease, MEK was hyper-active, suggesting it might be responsible for reducing adhesion among the keratinocytes to cause skin blistering. Inhibiting MEK (using a compound that is already FDA-approved for other diseases like melanoma) in the keratinocyte model restored their cohesion and suggests that targeting this pathway may be a novel treatment to halt skin blistering in Darier disease.
“It has been incredibly rewarding to see this work come together to advance our understanding of a rare disease that I actually see in my dermatology clinic. We’ve known for more than two decades that Darier disease is caused by mutations in a specific gene, yet we still have no approved treatments to offer patients, which is really frustrating to me as a physician-scientist. Our study is an important step in changing the way we think about treating Darier disease, potentially by repurposing MEK inhibitors that are already available in the clinic. I also hope our paper will highlight the need for more funding for research into rare diseases, which often get overlooked despite causing a lot of suffering and affecting a very large number of patients when all rare diseases are added up.”
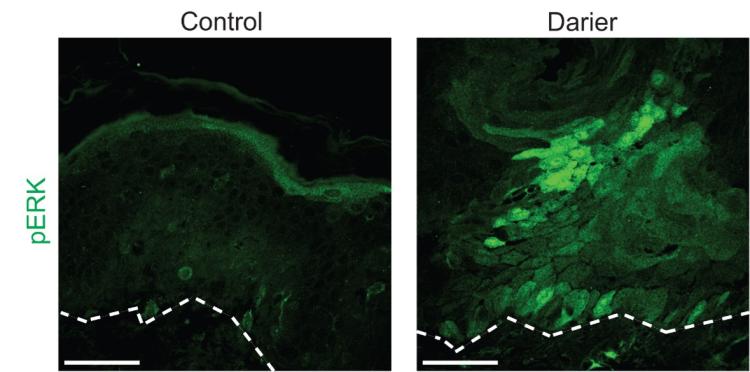
Paper Abstract: Mutation of the ATP2A2 gene encoding sarco-endoplasmic reticulum calcium ATPase 2 (SERCA2) was linked to Darier disease more than two decades ago; however, there remain no targeted therapies for this disorder causing recurrent skin blistering and infections. Since Atp2a2 knockout mice do not phenocopy its pathology, we established a human tissue model of Darier disease to elucidate its pathogenesis and identify potential therapies. Leveraging CRISPR/Cas9, we generated human keratinocytes lacking SERCA2, which replicated features of Darier disease, including weakened intercellular adhesion and defective differentiation in organotypic epidermis. To identify pathogenic drivers downstream of SERCA2 depletion, we performed RNA sequencing and proteomic analysis. SERCA2-deficient keratinocytes lacked desmosomal and cytoskeletal proteins required for epidermal integrity and exhibited excess MAP kinase signaling, which modulates keratinocyte adhesion and differentiation. Immunostaining patient biopsies substantiated these findings with lesions showing keratin deficiency, cadherin mis-localization, and ERK hyper-phosphorylation. Dampening ERK activity with MEK inhibitors rescued adhesive protein expression and restored keratinocyte sheet integrity despite SERCA2 depletion or chemical inhibition. In sum, coupling multi-omic analysis with human organotypic epidermis as a pre-clinical model, we found that SERCA2 haploinsufficiency disrupts critical adhesive components in keratinocytes via ERK signaling and identified MEK inhibition as a treatment strategy for Darier disease.